Introduction
The importance of osmosis is that is the impact the conveyance of supplements and the arrival of metabolic waste items. Living cells of the two plants and creatures are encased by a semipermeable film called the phone layer, which manages the progression of fluids and of breaking down solids and gases into and out of the phone. The film shapes a particular hindrance between the cell and its condition; not all substances can go through the layer with an equivalent office.
Osmosis
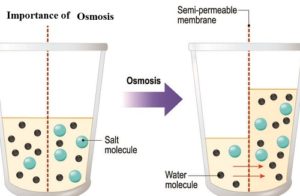
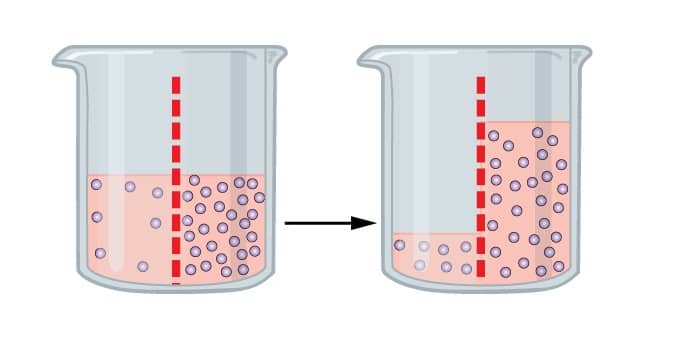
It is processed in which it tends the molecules of the solvent to pass a semipermeable film from a less gathered arrangement into a more.
Types of osmosis
- Exosmosis
- Endosmosis
Exosmosis
At the point when a substance is put in a hypertonic arrangement, the dissolvable atoms move outside the cell and the cell gets limp or experience plasmolysis, this is being called as exosmosis.
Endosmosis
At the point when a substance is put in a hypotonic arrangement, the dissolvable atoms move inside the cell and the cell gets bloated or experience Deplasmolysis, This is known as endosmosis.
Types of Osmotic solutions
- Isotonic Solution
- Hypertonic Solution
- Hypotonic Solution
Isotonic Solution
An isotonic is when a successful osmole focus is equivalent to that of another arrangement. The arrangements on either side of a cell film are isotonic if the grouping of solutes outside the cell is equivalent to the convergence of solutes inside the cell.
Hypertonic Solution
A hypertonic arrangement is one that has a higher solute focus outside the cell than inside.
Hypotonic Solution
A hypotonic arrangement is the one that has a higher solute focus inside the cell than outside.
Impotance of osmosis

It is liable for the assimilation of water from the dirt and directing it to the upper pieces of the plant through the xylem, Assimilation impacts the vehicle of supplements and the arrival of metabolic waste items. It keeps up the bloat of cells, It balances out the interior condition of a living being by keeping up the harmony among water and intercellular liquid levels. This cycle controls the cell to cell dispersion of water.It is a cycle by which plants keep up their water content regardless of the steady water misfortune because of happening. This cycle controls the cell to cell dispersion of water. Assimilation additionally controls the dehiscence of foods grown from the ground. Higher osmotic weight secures the plants against dry season injury. Assimilation initiates cell turgor which directs the development of plants and plant parts.
Osmotic pressure
Osmotic pressure is the compel required to prevent water from diffusing through a film as a natural side effect. It is dictated by the convergence of the solute. Water diffuses into the territory of higher fixation from the zone of lower focus. At the point when the centralization of the substances in the two territories in contact is extraordinary, the substances will diffuse until the focus is uniform all through.
Osmotic weight can be determined utilizing the condition:
Π=MRT
where Π indicates the osmotic weight,
M is the molar convergence of the solute,
R is the gas consistent,
T is the temperature
the osmotic pressure is

Side effects of Osmosis
The plant cell has thick dividers and requires more water. osmosis influences the cells in an unexpected way. A creature cell will lyse when put in a hypotonic arrangement contrasted with a plant cell. The cells won’t burst when put in a hypotonic arrangement. Actually, a hypotonic arrangement is ideal for a plant cell. A creature cell endures just in an isotonic arrangement.
The osmotic stream can be halted or turned around, additionally called switch assimilation, by applying an outside strain to the sides of the solute. The base compel required to stop the dissolvable exchange is known as the osmotic weight.
Conclusion
Assimilation is significant for the cells for some reasons.The supplements, water and different solutes move all through the cell by the cycle of assimilation. It helps in the development of significant materials all around of the cell.