List of Thermal Properties of matter
The thermal properties of matter is a vast area comprising a number of topics. In this context, we will come across various topics related to the thermal properties of matter that play a very vital role in understanding the concept.
Before understanding the thermal properties of matter, let us first get some knowledge about some preliminary topics. The first parameter that constitutes the thermal properties of the matter is temperature. It is the measure of the degree of hotness or coldness of a body. The SI unit of temperature is Kelvin (K), while Centigrade (°C ) is the most commonly used unit for temperature.
Since that deals basically with the measurement of the temperature of a body, the branch is called thermometry. Hence, the instrument used to measure temperature is called a thermometer. At home, generally, a mercury thermometer is used.
The temperature rises or falls in accordance with the motion of the atoms in the substance. If there is an increase in the random motion of the constituent atoms of the substance, then there is a rise in temperature.
On the other hand, if the random motion decreases, the temperature of the substance falls. It is known that the motion of atoms is a consequence of Kinetic Energy. The increase in Kinetic Energy increases the atoms’ random motion, and the decrease in Kinetic Energy decreases it. Hence, the temperature can be defined as the average KE of the substance.
Having set the base, we can now move forward with the thermal properties of matter in detail. Heat is a form of energy. Heat flows from a hot body to a cold body. It means that heat flows due to the difference in temperature between the two bodies. The SI unit of heat is Joule (J), and the other unit used is Calorie (cal). The relation between the units is given by the equation : 1 cal = 4.182 J.
Boyle’s Law –
At a constant temperature, the volume of a gas is found to be inversely proportional to its absolute pressure. It is given as,
P1V1 = P2V2
The absolute temperature has its 0 K at – 273 °C . Hence absolute zero is, 0 K = – 273 °C .
Charles’ Law –
At constant pressure, the volume of a gas is found to be directly proportional to its absolute temperature. It is given as,
V1/T1 = V2/T2
The most fundamental part associated with the thermal properties of matter is the Ideal Gas Equation, a combination of Boyle’s Law and Charles’ Law, which is given by PV=nRT
P is the gas’s pressure, V is the volume, n is the number of moles of the gas, R is the Universal Gas Constant, and T is the temperature.
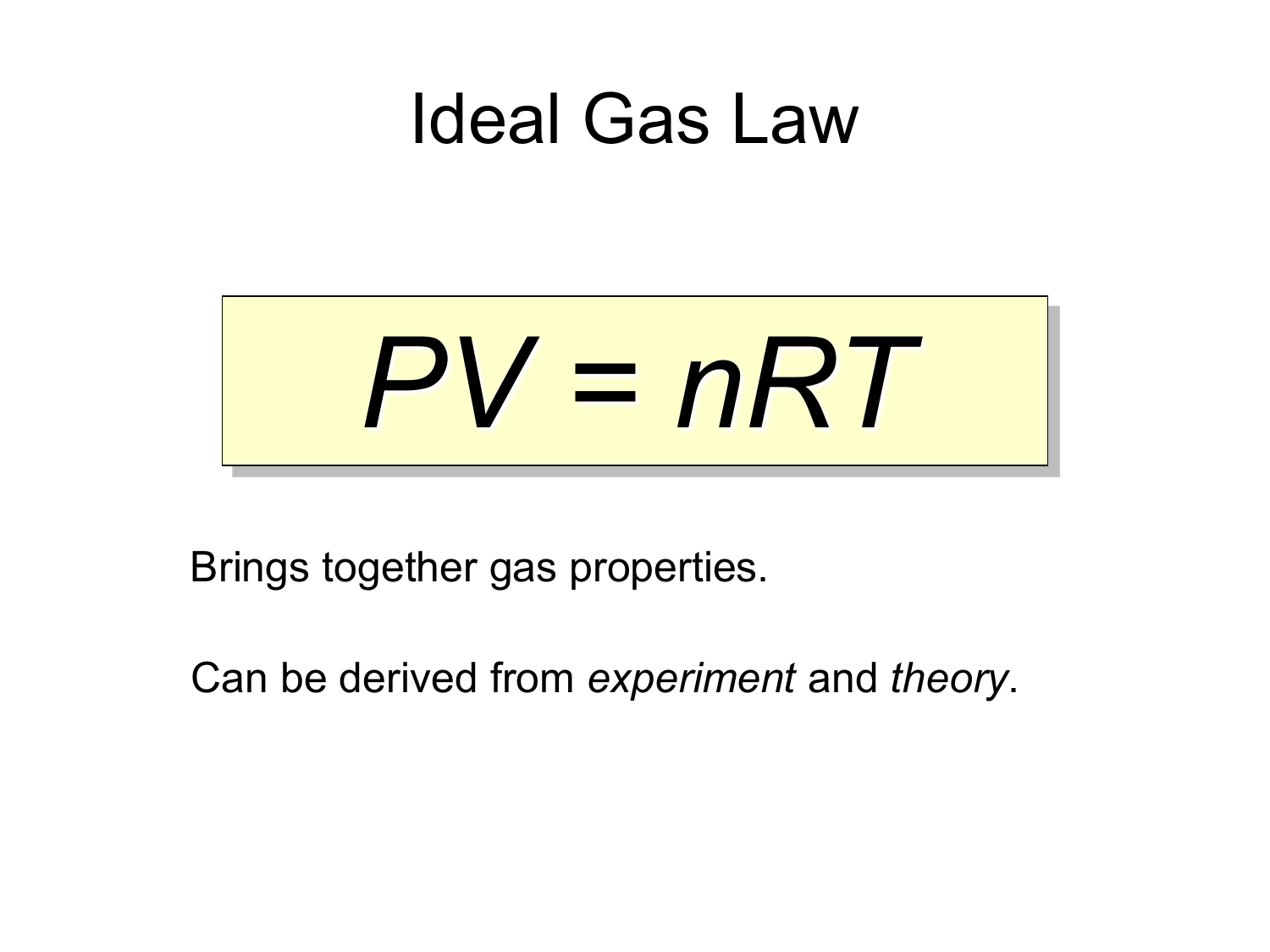 The Ideal gas equation is purely hypothetical because no gas behaves ideally, and there is always some distortion from the ideal behaviour. Hence, we can define ideal gas as the gas that obeys the Ideal Gas Equation.
The Ideal gas equation is purely hypothetical because no gas behaves ideally, and there is always some distortion from the ideal behaviour. Hence, we can define ideal gas as the gas that obeys the Ideal Gas Equation.
Kinetic Theory of Gases
According to the Kinetic Theory of gases, a gas consists of tiny molecules that do not interact with one another (except at the time of collision). The molecules are considered to be far apart in order to make them stay in constant motion.
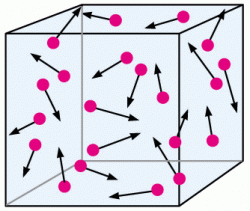
The absolute zero has a significant part in the Kinetic theory of gases, as it is that temperature at which all molecular motion ceases to exist or the constant motion of the molecules of the gas stops. The equation is,
KE(avg.) = (3/2) * k *T
where k is Boltzmann’s constant.
Now comes Heat Capacity and Specific Heat Capacity, another important aspect of the thermal properties of matter. Heat capacity for any substance can be defined as the amount of energy required to raise that substance’s temperature by 1 Kelvin ( or 1°C ). It is given by,
Heat Capacity = Q/(T2 – T1)
Q is the amount of heat energy while (T2 – T1) changes in temperature. The SI unit of Heat Capacity is J/K.
On the other hand, Specific Heat Capacity can be defined as the amount of heat energy needed to produce a unit rise in temperature in the material’s unit mass. It is given by,
Specific Heat Capacity = (Q2 – Q1)/m*(T2-T1)
Where (Q2 – Q1) is the amount of heat, m is the mass, and (T2 – T1) is the temperature change. The SI unit of Specific Heat Capacity is J/kg K.
Thermal Expansion is another very important part of the thermal properties of matter. By the name, thermal expansion is the increase in the body’s size due to the body’s temperature increase. Now, this expansion may be linear (increase in length), superficial (increase in area), or volume expansion (increase in volume).

The next constituent of the thermal properties of matter is thermal stress. Thermal stress is caused by the change in temperature. Suppose a rod is held in between two fixed supports, and the temperature is increased. The rod will naturally tend to expand, but it experiences some stress because of the fixed supports. This stress is called thermal stress. It is given by,
Thermal Stress = F/A = Y*α*(T2 – T1)
where Y is the Young’s Modulus, α is the coefficient of linear expansion, F is the force on the body, A is the area, and (T2 – T1) is the change in temperature.
Calorimetry
Calorimetry, the interesting as well as the important part of the thermal properties of matter, is mainly associated with the measurement of heat. The apparatus required to serve the purpose is called a calorimeter. Calorimetry is based on the principle,
Heat lost by the hot body = Heat gained by the cold body.
The above equation is also called the law of energy conservation.
Important points from the law:
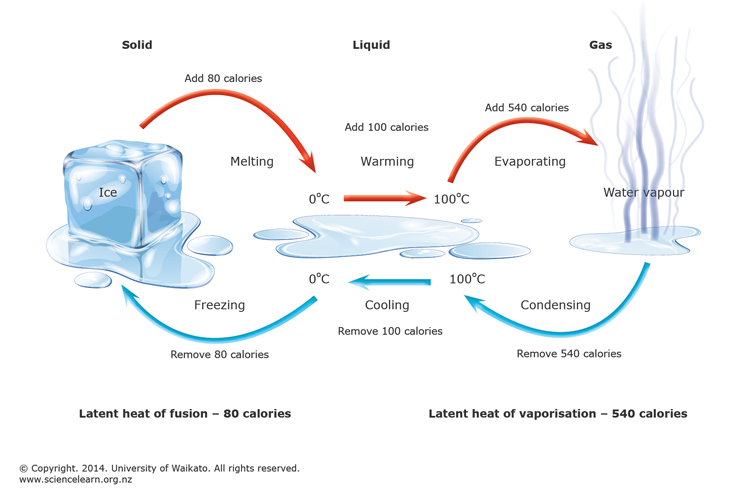
- Change of state – Transition of matter from one state to another is termed a state change.
- Melting is the process of change of state from solid to liquid, and Fusion is the process of change of state from liquid to solid.
- The temperature at which the solid melts is called the melting point. The temperature remains constant until the entire amount of the substance melts.
- The state change from liquid to gas is called evaporation, and the state change from gas to liquid is called condensation.
- The state change directly from solid to gas without forming liquid is called sublimation.
Some more terms associated with the thermal properties of matter
Heat formula – Q = m*s*t, where Q is the amount of heat required, m is the mass, s is the specific heat capacity, and t is the temperature.
Latent Heat – is the amount of heat required to change the state of a unit mass of a substance.
Heat Transfer – heat can be transferred between bodies in 3 ways – conduction, convection, and radiation.

Let us now understand each of the processes separately:
Conduction – It is the mechanism of heat transfer from one body to another in contact due to the difference in temperature. Gases are considered to be poor conductors of heat in comparison to liquids.
Applications of Conduction :
- Bad conductors of heat are used for insulation purposes.
- Heaters are made from the known good conductors of heat.
- To prevent heat loss to the environment, thermal insulators are used.
Convection – Convection is the process of heat transfer that involves the actual motion of matter from one body to another. This process is hence possible only in fluids. Convection is of two types – natural convections and forced convection. The land breeze and night and Sea breeze in the morning is caused due to the process of natural convection.
Applications of convection : The process of convection is utilised in,
- Air conditioner
- Refrigerator
- Electric Fan
- Land breeze
- Sea breeze
Radiation – As we have seen that both convection and conduction require a medium to facilitate heat transfer. On the other hand, radiation is the direct heat transfer process from one body to another without any intervening medium.
We can understand radiation by a simple example. Suppose you are sitting by a camp fire. After sitting for some time, you feel warm. This is because heat is radiated by the campfire to your body by the process of radiation; definitely, it required no medium.
Applications of radiation :
- Thermal Radiators
- The bottom of cooking utensils are made black to radiate heat into the food being cooked.
Thermal Resistance – the opposition faced by the heat flow from the body is termed thermal resistance.
thermal resistance – l/KA
where l is the length, K is the thermal conductivity, and A is the area.
Newton’s Law of Cooling is an important concept of the thermal properties of matter. It states that the heat loss rate from a body is directly proportional to the difference in temperature (less than 40 °C). It is given by:
dT/dt = -K(T – Ts)
Black body Radiation
Now let us understand the difficult but interesting constituent in the thermal properties of matter, called the Black body radiation. The black body is an ideal body that happens to absorb all the electromagnetic radiation, incident on it, completely. The radiation radiated from this black body is termed as Black body radiation.
Terms related to black body radiation:
- Emissive Power – Amount of energy radiated per unit area from the surface of a black body, per unit time, and per unit wavelength. Its SI unit is Js-1 m-2 .
- Absorptive Power – The ratio of the amount of heat absorbed by a body to the total heat incident.
- Perfect Black Body – A body is said to be a perfect black body if its absorptivity is 1. It means this body neither transmits nor reflects the energy incident on it but only absorbs all of it.
- Wein’s Displacement Law – It states that the wavelength of radiation curves from the black body is inversely proportional to the temperature. It is given by Wavelength = b/T where b is the Wein’s displacement constant, and T is the absolute temperature.
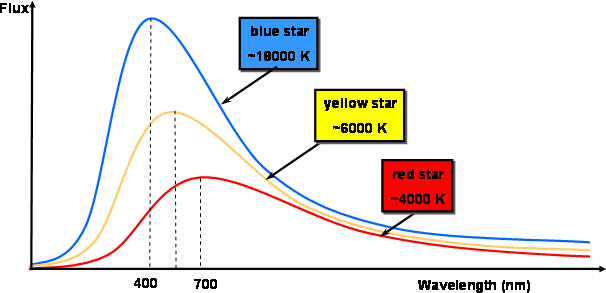
- Stefan’s Law – It states that thermal radiations emitted from a black body is directly proportional to the area multiplied with the fourth power of absolute temperature.
This was about the theory related to the thermal properties of matter now, let us look at the problems.
Thermal Properties of matter Questions & Answers
Question 1. A circular hole of diameter 3 cm is made in an aluminium plate at 0 0 C .what will be the diameter at 1000 C?
Linear expansion for aluminium = 2.3 * 10-3 / 0 C
Solution:
Diameter of the circular hole in aluminium plate at 00 C=3 cm
With an increase in temperature from 00 C to 1000 C diameter of the ring increases
using
L=L0(1+αΔT)
where L0=3 cm
α = 2.3 * 10-3 / 0 C
ΔT=(100 -0)=100 0 C
we can find diameter at 1000 C
L=3(1+2.3*10-3*100)
=3.69 cm
Question 2. What is Specific Heat Capacity?
Solution : Specific Heat Capacity can be defined as the amount of heat energy needed to produce a unit rise in temperature in the material’s unit mass. It is given by,
Specific Heat Capacity = (Q2 – Q1)/m*(T2-T1)
Where (Q2 – Q1) is the amount of heat, m is the mass, and (T2 – T1) is the temperature change. The SI unit of Specific Heat Capacity is J/kgK.
Question 3. What is the difference between heat and temperature?
Solution : Temperature is defined as the measure of hotness or coldness of a body. Its SI unit is Kelvin (K).
Heat is a form of energy. Its SI unit is Joule (J).
Question 4. State the principle of calorimetry.
Solution : The amount of heat lost by the cold body is always equal to the amount of heat gained by the cold body at equilibrium.
That means Heat lost by one body = Heat gained by another body.
Question 5. If a is coefficient of Linear expansion, b coefficient of superficial expansion, c coefficient of Volume expansion. Which of the following relations is true?
a. b=2a
b. c=3a
c. b=3a
d. a=2b
Solution : c is the correct answer
Question 6. Two thermometers are constructed in the same way except that one has a spherical bulb and the other an elongated cylindrical bulb. Which of the two will respond quickly to temperature changes?
Solution : A cylindrical bulb has a greater surface area than a spherical bulb of the same volume. Hence the thermometer with an elongated bulb will respond to temperature changes more quickly than the one with a spherical bulb.
Question 7. State the Significance of absolute 0 in Kinetic Theory of gases?
Solution : Absolute Zero is the temperature at which all molecular motion ceases to exist.
Question 8. What is a perfect black body?
Solution : A body with absorptivity 1 is called a perfect black body. Such a body neither transmits nor reflects the heat but only absorbs it completely.
Question 9. Two gases are mixed. The pressure of gas A is 72 pa. The volume of gas B is 4 times the volume of gas A. What is the pressure of gas B?
Solution : Let the Volume of Gas A(V1) = x
The volume of gas B (V2) = 4x
Pressure of gas A (P1) = 72 pa
Pressure of gas B (P2) = ?
By using Boyle’s Law,
P1V1 = P2V2
=> 72 * x = P2 * 4x
=> P2 = 72/4 = 18 pa
Therefore, the pressure of gas B is 18 pa.
Question 10. State the relation of absolute zero scales with the centigrade scale.
Solution : The relation is given by, 0 K = – 273 °C .
Question 11. How is Kinetic Energy related to the absolute temperature?
Solution : The random motion of atoms is caused by a change in Kinetic Energy. When the Kinetic energy increases, the random motion of atoms also increases while the random motion decreases when there is a decrease in the kinetic energy. The increase in random motion causes a rise in temperature, while the decrease in random motion causes a fall in temperature of the substance. Hence, it can be concluded that the increase in Kinetic energy increases the temperature of the substance and vice – versa. This is how Kinetic energy is related to the absolute temperature.
Question 12. Give reasons for the following:
a. Light coloured clothes are preferred in summers.
b. The earth without an atmosphere would be extremely cold.
Solution :
a. Light coloured clothes are preferred in summers because they are bad conductors of heat. They radiate away from the heat incident on them, and thus, one wearing light coloured clothes does not feel hot in summers.
b. The atmosphere of the earth helps in trapping the heat radiated from the sun. It reflects the infrared rays and traps the rest of the heat. If the atmosphere is not present on earth, no heat would be trapped, that means all the heat radiated from the sun would be lost, and thus earth will be very cold.
This is about the thermal properties of matter. I hope now you have grasped a lot on this topic.
– Sweta Upadhyay
